Results To Date
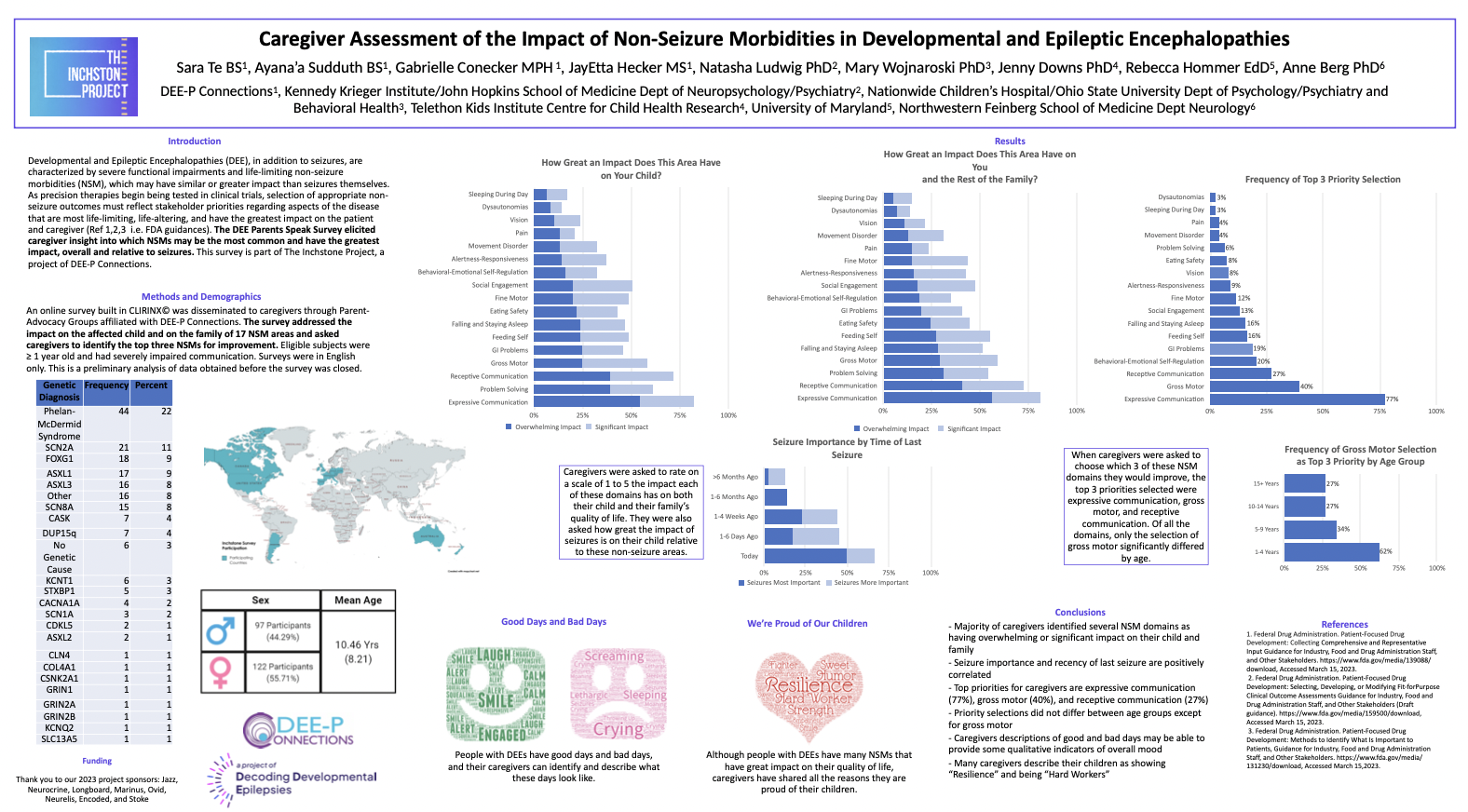
Preliminary Results from groundbreaking DEE Parents Speak Survey Shared with the Community
On this community update, The Inchstone Project Research Team shares progress on our efforts to create a range of assessment tools that can accurately measure progress in those more profoundly impacted by disorders and are consistently left unmeasured. In particular, the team provides an update on the data collected via our recent DEE Parents Speak survey from nearly 270 families on what their children CAN do, what their priorities are and results on how well the tools we are using capture the abilities of those profoundly impacted by DEEs.
Presentations at International Neuropsychological Society 2024
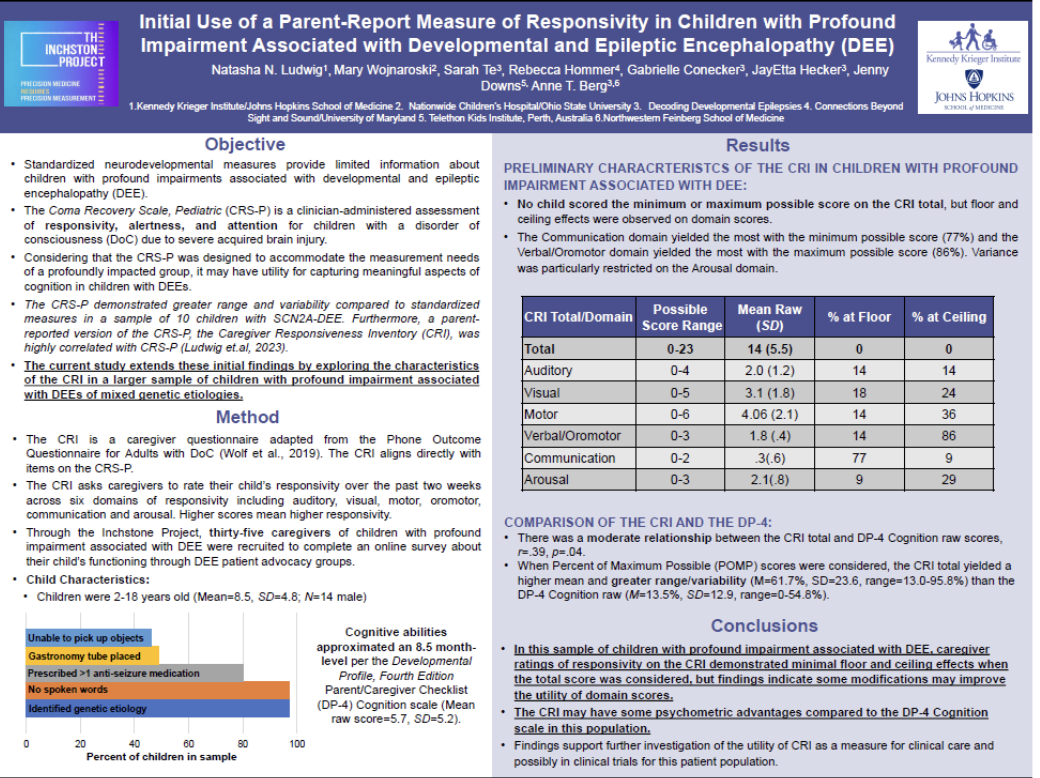
Two Inchstone posters were orally presented at the International Neuropsychological Society (INS) meeting as part of an Early Life Epilepsy poster symposium of the Epilepsy SIG - one by Dr. Mary Wojnaroski (right) and another by Dr. Natasha Ludwig (left).

Two Seminal Publications
Two groundbreaking publications were released in late 2023 and early 2024 - each contributing to a shift in the conversations about why we must and how we can measure those profoundly impacted by DEEs and other severe disorders. A paper of the FDA Patient Focused Drug Development process and how it should consider the DEEs by Dr. Anne Berg is on the left. Another on the use of meaningful change to measure progress in the DEEs by Dr. Jenny Downs is on the right.
Poster presented at American Epilepsy Society Meeting - 2023
The Incshtone Project Research Team presented some early results of our DEE Parents Speak survey of caregivers across the DEE community sharing what their priorities are for their children, what abilities they have and testing a range of clinical assessment tools across more than 20 disorders.
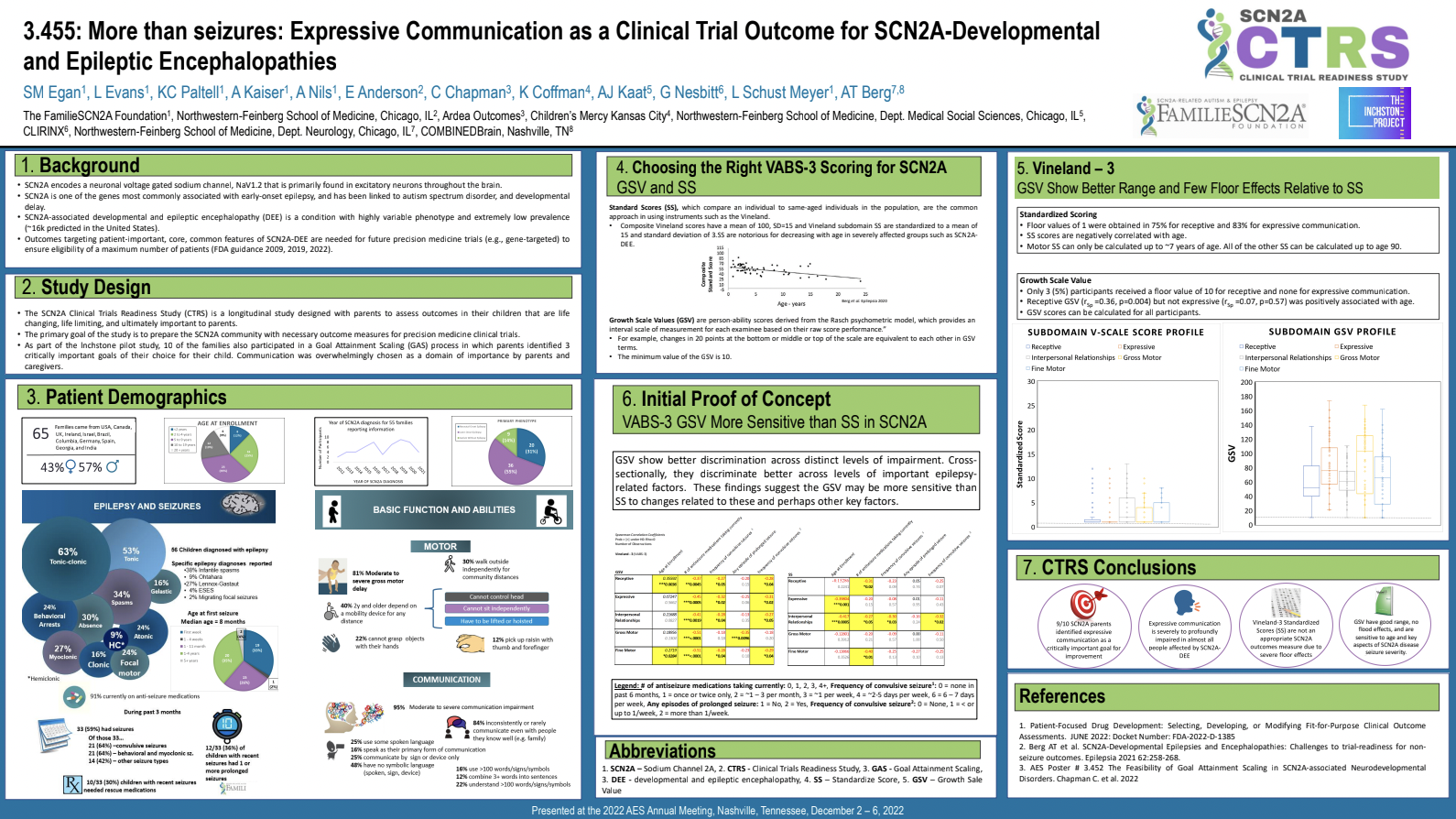
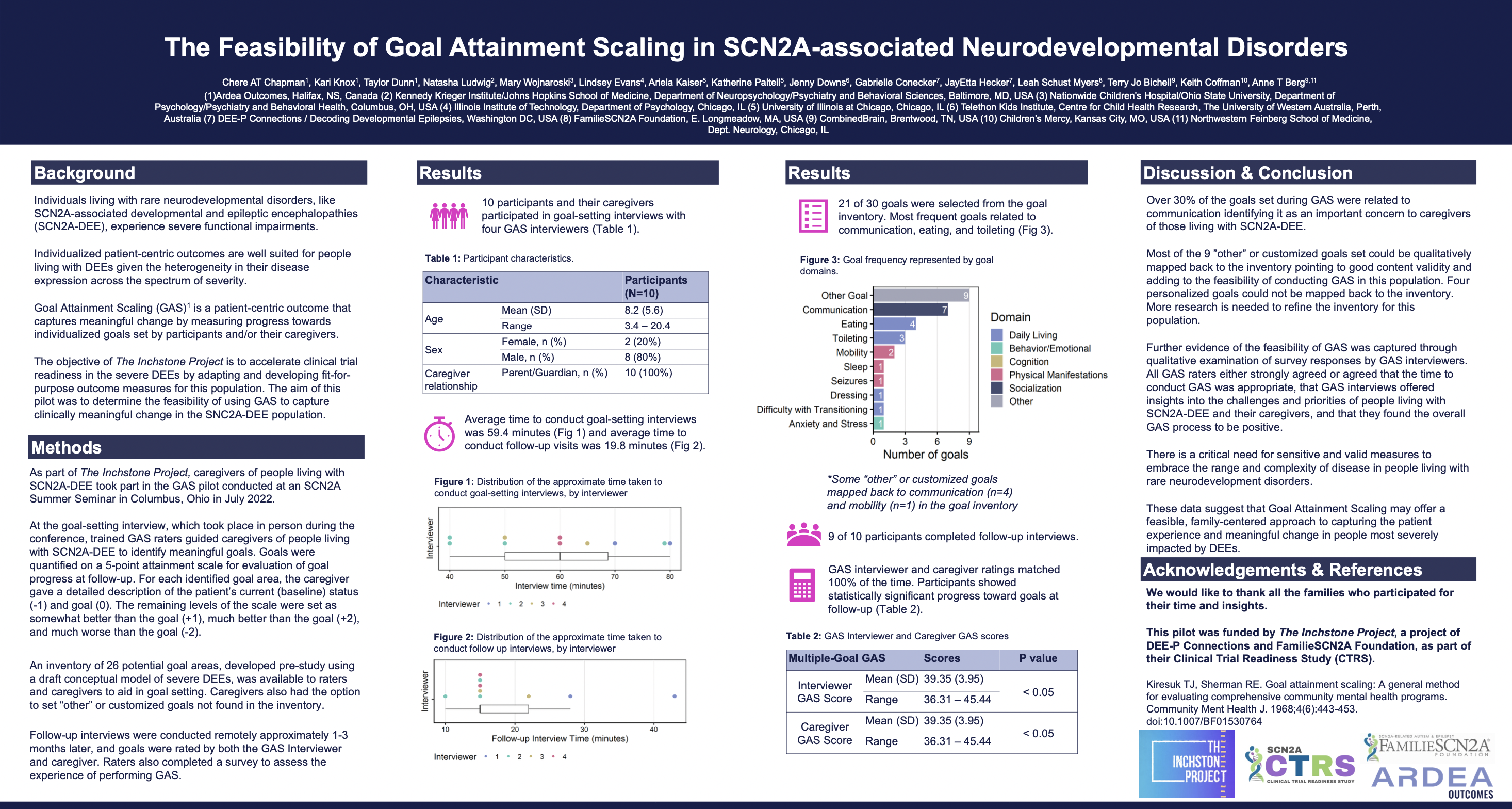
Hosted Symposium at Gatlinburg 2023 - "Capturing Inchstones: Novel Approaches to Measurement for Severely Affected Children With SCN2A-Associated Developmental and Epileptic Encephalopathy"
This symposium shared results from our pilot study conducted with the FamilieSCN2A Foundation. The Inchstone Project and the SCN2A Clinical Trial Readiness Study played a critical role in designing, executing and supporting the collaborative, interdisciplinary, and multi-center research presented at this symposium. Leah Schust Myers and Gabi Conecker, Executive Directors of the FamilieSCN2A Foundation and The Inchstone Project and the International SCN8A Alliance respectively, were discussants who shared their lived experience as advocates and parents of children with severe DEEs. This was followed by presentations about measuring Minimally Clinically Important Difference, the use of individualized outcomes using Goal Attainment Scaling and the novel use of responsivity measures in those profoundly impacted by disease.



Four Latebreaker Posters Presented at AES 2022
In 2022, The Inchstone Research Team conducted a pilot study in conjunction with our partners at FamilieSCN2A Foundation to test the utility of current assessment tools with those profoundly impacted by DEEs. The results of this study had a 100% acceptance rate for late-breaking abstracts submitted to the AES conference in 2022! Click on each poster to enlarge them.
Members of our team helped plan and presented at an Investigator Workshop on Non-Seizure Outcome Measures for DEEs - AES 2022
Clinically meaningful, validated outcome measures are critical for the development of novel therapeutics. Seizures as a
primary outcome may not always be suitable or sufficient for patients with Developmental and Epileptic Encephalopathies
(DEEs). This workshop will present an overview of challenges in evaluating outcomes in DEEs, the state of existing and
emerging measures and tools appropriate for DEEs, discuss development of new measures and refinement of existing
measures to fit patients with DEE, and highlight patient/ caregiver-led research efforts to accelerate urgently needed
improvements. Panelists include speakers from diverse backgrounds, experience, and roles.



Submitted extensive feedback to the FDA on their Draft Guidance on Developing or Modifying Clinical Outcome Assessments
We submit these comments as a diverse group of stakeholders dedicated to improving outcome measures for those with severe developmental and epileptic encephalopathies (DEEs) and considerable medical complexity. We collaborate through The Inchstone Project (housed within DEE-P Connections)—a group of leading clinical outcome assessment (COA) researchers, clinicians, patient advocacy groups, and industry partners—with the goal of ensuring that there are outcome measures that can capture even the most minute progress by the most severely impacted individuals - the inchstones, not milestones. We believe that with the emergence of genetic therapies and the need to include those significantly impacted by these rare disorders in clinical trials, precision medicine requires precision measurement.
Our comments provide a brief summary of the challenges patients with DEEs face and outline specific aspects of the guidance which could better reflect the distinct needs and challenges of measurement in this population. We highlight the need for accommodation to assure their equitable inclusion in both COAs and more importantly, the drug development and evaluation process.